killerred蛋白能看到荧光吗
KillerRed是个完全由基因编码的光毒性红色荧光蛋白,可接受绿色光照(540~580nm)生成活...
2024-04-29作者:激发光源事业部时间:2019-10-11 16:52:17浏览11879 次
经常有客户咨询BFP(Blue Fluorescent Protein)的激发波长和发射波长,现在将BFP的激发波长和发射波长的光谱图提供给大家参考。BFP的激发波长在400nm左右,发射波长在450nm左右。
美国路阳生产一款便携式荧光蛋白激发光源,能够方便快捷观察EGFP在动植物体内的表达。我们免费提供样机供科研院所试用,满意付款。如有兴趣,可添加微信号(luyor01)联系。

EGFP在烟草上的表达
绿色荧光蛋白在果蝇上的表达
经常有客户咨询BFP蓝色荧光蛋白(Blue Fluorescent Protein)的激发光波长和发射光波长,现在将BFP蓝色荧光蛋白的激发光波长和发射光波长的光谱图提供给大家参考。
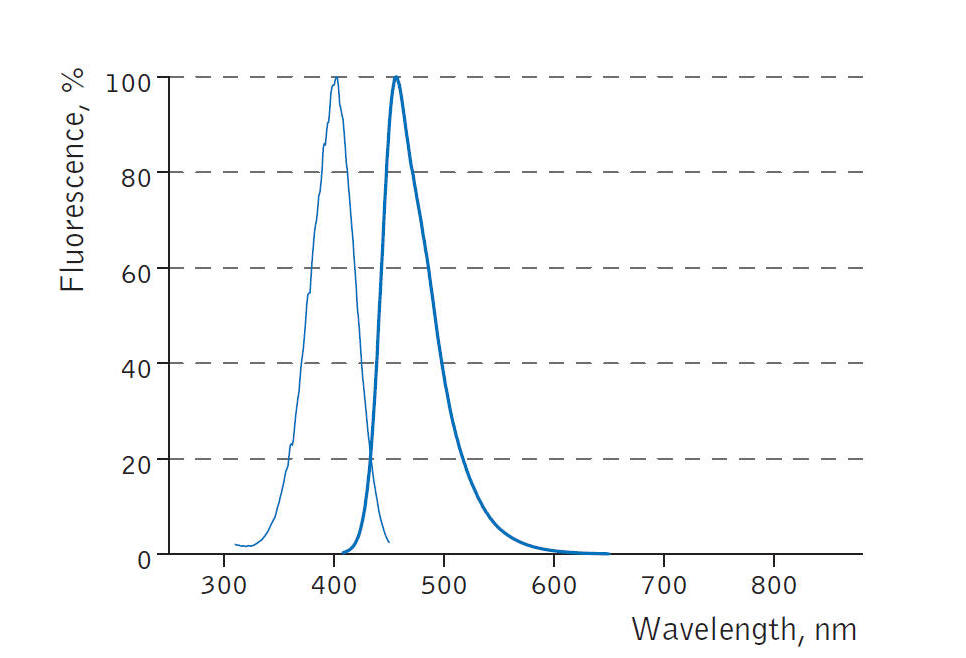
BFP蓝色荧光蛋白的激发和发射光谱图
如果要观察BFP蓝色荧光蛋白(Blue Fluorescent Protein)的表达,美国路阳生产的便携式荧光蛋白激发光源可以选择LUYOR-3260VI和LUYOR-3415VX系列双波长荧光蛋白激发光源。蓝色荧光蛋白采用美国路阳紫光激发,佩戴LUV-20A绿色观察眼镜观察,如希望提供更多详细信息,可直接联系上海路阳生物技术有限公司的销售客服。

| 荧光蛋白名称 | 激发光波长nm | 发射光波长nm |
|---|---|---|
| TagBFP | 402 | 457 |
| mTagBFP2 | 399 | 454 |
| Azurite | 383 | 450 |
| EBFP2 | 383 | 448 |
| mKalama1 | 385 | 456 |
| Sirius | 355 | 424 |
| Sapphire | 399 | 511 |
| T-Sapphire | 399 | 511 |
蓝色和青色荧光蛋白变种是将绿色荧光蛋白的66位的酪氨酸残基突变为组氨酸形成的。这一转变使蓝色发射光更大波长为450nm,而突变为色胺后,荧光的峰值在480nm。这两个荧光蛋白的荧光都很弱,需要改造来提高折叠效率和荧光亮度。尽管进行了改造,两者的荧光蛋白亮度仅为增强型绿色荧光蛋白的25-40%。除此之外,蓝色和青色荧光蛋白在不常用的光谱区应用起来很有效,所以需要特殊的滤光片和激光光源。
除了以上缺点,在多颜色荧光标记和FRET实验中这两种荧光蛋白得到了较多的应用。尤其是增强型青色荧光蛋白,可以被氩离子激光激发脱离峰值(用457nm光谱线),而且比蓝色衍生物更能抵抗光漂白作用。跟其他荧光蛋白相比,对可见光蓝色区域的荧光蛋白并没有引起很高的重视,在这一组荧光蛋白中主要研究集中在青色荧光蛋白中。
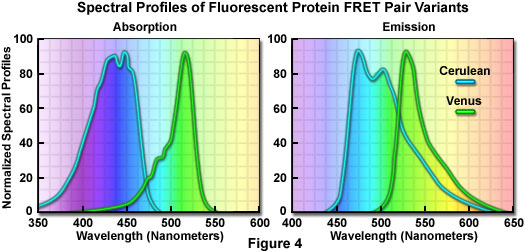
在所有改进过的青色荧光蛋白中,AmCyan1和Cerulean两个变种最有应用前景。AmCyan1荧光蛋白变种来源于Anemonia majano。跟增强型青色荧光蛋白在哺乳动物表达中相比,已经人源化产生了亮度相对高并可以抵抗光漂白的荧光蛋白。与其他的珊瑚蛋白相似,这种荧光探针也易于形成四聚体。Cerulean荧光探针已经通过对增强型青色荧光蛋白定点突变开发出来,产生了一个高消光系数和量子产量提高的变种。Cerulean的亮度至少为增强型青色荧光蛋白的两倍;在TRET中,也证实与黄色荧光蛋白一起使用时,大大提高了信噪比。
Fluorescent proteins emitting in the blue region of the visible light spectrum (approximately 440 to 470 nanometers) were first obtained from site-directed mutagenesis efforts targeted at the tyrosine amino acid residue at position 66 in the GFP chromophore (see Figure 2). Conversion of this residue to histidine (Y66H) produces a blue fluorescent protein (BFP) that exhibits a broad absorption band in the ultraviolet centered close to 380 nanometers and an emission maximum at 448 nanometers. The original protein exhibited only about 15 to 20 percent of the parent GFP brightness value due to a low quantum yield and required additional secondary mutations to increase its folding efficiency and expression levels. Subsequent investigations and several additional mutations led to an enhanced BFP version that is still only 25 percent as bright as the enhanced green variant and displays limited photostability compared to many other fluorescent proteins.
The primary motivation for developing blue fluorescent proteins in the mid-to-late 1990s was the keen interest in creating matched pairs for fluorescence resonance energy transfer (FRET) experiments and multicolor labeling. Because the spectral characteristics (fluorescence emission profile) of BFP are readily distinguishable from EGFP, this protein combination was one of the first utilized for multicolor imaging. Blue fluorescent protein also has the distinction of being incorporated into the first genetically encoded biosensor along with an enhanced GFP variant to demonstrate FRET through linkage of the two fluorescent proteins via an intervening protease-sensitive spacer. The broad emission peak of BFP overlaps to a significant extent with the excitation spectrum of red-shifted GFP variants to yield a Förster distance of 4.1, a reasonable value for measuring FRET. Blue fluorescent protein has also been coupled with several GFP derivatives into biosensors designed to monitor transcription factor dimerization, calcium, and apoptosis.

Aside from the limited brightness levels and rapid photobleaching, blue fluorescent proteins also suffer from the fact that they must be excited with ultraviolet light, which is phototoxic to mammalian cells, even in limited doses. Furthermore, working in this spectral region is often hampered by autofluorescence and high absorption levels by cells and tissues, as well as light scattering. Microscopes operating in the ultraviolet also require specialized light sources, optics, and filter combinations that further complicate imaging. For all of the reasons listed above, the quest for more efficient blue fluorescent proteins has only been pursued by a few research groups. Investigations of mutagenesis using non-natural amino acids positioned in and around the chromophore have led to several blue-shifted "artificial" fluorescent protein variants that may find utility in several biological and photophysical applications.
Illustrated in Figure 2 are the chromophore structures for color variants of green fluorescent protein. In all cases, the first step in maturation of the chromophore is a series of torsional adjustments that relocate the carboxyl carbon of the amino acid at position 65 so that it is in close proximity to the amino nitrogen of the glycine residue at position 67 (Gly67) in the polypeptide backbone. Nucleophilic attack by this carbon atom on the amide nitrogen of glycine, followed by dehydration, results in formation of an imidazolin-5-one heterocyclic ring system. Fluorescence occurs when oxidation of the aromatic amino acid (position 66) carbon bond by molecular oxygen extends electron conjugation of the imidazoline ring system to include the aromatic substituent. The cyan, green, and yellow fluorescent protein variant chromophores are discussed in greater detail in the following sections.